Abstract
Lipopolysaccharide (LPS) is an inducer of interleukin (IL)-18, which in turn plays important roles in immune responses. Previously, we reported that tumor necrosis factor (TNF)-a production could be detected in human peripheral blood mononuclear cells (PBMCs) treated with relatively low concentration of LPS (1 ng/ml), but that same concentration of LPS could not induce IL-18 production. In the present study, we found that LPS at relatively high concentrations (10 – 1000 ng/ml) induced IL-18 production in a concentration-dependent manner both in monocytes isolated from PBMC, and that histamine (10—7 to 10—4 M) inhibited IL-18 production induced by LPS. The studies using receptor subtype-selective agonists and antagonists suggested that the effect of histamine was mediated by H2 receptor but not by H1, H3 and H4 receptors. Therefore, the stimulation of H2 receptor might be beneficial in the treatment of sepsis through inhibiting LPS-elicited IL-18.
Keywords: Human; PBMC; Monocyte; IL-18, LPS; Histamine; H2 receptor; Bucladesine Dibutyryl-cAMP
Introduction
Serious infections with Gram-negative bacteria can develop sepsis syndrome, a hyper-inflammatory condition that is largely induced by lipopolysaccharide (LPS)-induced secretion of pro-inflammatory cytokines. LPS, a potent activator of innate immunity, interacts with host immune cells either by binding to surface CD14 molecules or by forming a complex with soluble CD14 and binding to the cognate sites on monocytes or macrophages, Toll-like receptor (TLR)-4. LPS induces the elevation of IL-1hconverting enzyme or caspase-1. IL-18 is synthesized as a precursor protein that requires cleavage with caspase-1 for activity as in the case of IL-1h, and secreted from LPS-activated monocytes or macrophages. IL-18 with IL-12 synergistically produced IFN-g in T-cells and monocytes in which IL-12 has been shown to upregulate the h-subunit of IL-18 receptor complex.
Histamine participates in vasodilatation, smooth muscle contraction, mucus hypersecretion, and edema formation. Histamine also presents immunoregulatory properties as it modulates cytokine production by different cell types. Histamine exerts its effects through the stimulation of H1, H2, H3 and H4 receptors. H2 receptor stimulation is coupled with the activation of adenylate cyclase and the cyclic adenosine monophosphate (cAMP) protein kinase A pathway in U937 cells derived from the monocyte or macrophage lineage. It was reported that histamine, via H2 receptors, suppresses IL-12 production and stimulates IL-10 secretion in LPS (1000 ng/ml)-treated peripheral blood mononuclear cell (PBMC), In the previous study, we found that histamine suppressed LPS (1 ng/ml)-induced tumor necrosis factor (TNF)-a production as well as the expression of ICAM-1 and B7.1 via H2 receptor in PBMC. Because the production of IL-18 was not detected in LPS (1 ng/ml)-treated PBMC, the effect of histamine on the production of IL-18 is not analyzed yet. In the present study, we found that high concentrations of LPS (10 – 1000 ng/ml) induced the production of IL-18 in monocytes. Under such concentrations, we examined the effects of histamine on the LPS-induced IL-18 production and found, for the first time, the strong inhibiting effect of histamine on IL-18 production through stimulation of H2 receptors.
Materials and methods
Reagents and drugs
LPS from Escherichia coli (L8274, serotype 026:B6) was purchased from Sigma (St. Louis, MO). Pure water produced by MILLIPORE (MILLIPORE JAPAN, Tokyo, Japan) was the solvent solution for LPS. Histamine was purchased from Nakalai Tesque, Inc. (Kyoto, Japan). Dimaprit, 4-methylhistamine, and 2-(2-pyridyl)-ethylamine dihydrochloride were kindly donated by Drs. WAM Duncan and DJ Durant (The Research Institute, Smith Kline and French Laboratories, Welwyn Garden City, Herts, UK). R-(a)-Methylhistamine dihydrochloride was a gift from Dr. J-C Schwartz (the Unite de Neurobiologie, Centre Paul Broca de l’INSERM, Paris). d-Chlorpheniramine maleate, ranitidine, and famotidine were provided by Yoshitomi Pharmaceutical Co. Ltd. (Tokyo, Japan), Glaxo Japan (Tokyo, Japan), and Yamanouchi Pharmaceutical Co. Ltd. (Tokyo, Japan), respectively. Thioperamide hydrochloride was provided by Eisai Co. Ltd. (Tokyo, Japan). Dibutyryl cAMP (dbcAMP) was purchased from Wako Pure Chemical Ind. Ltd. (Tokyo, Japan).
Preparation of monocytes
Normal human PBMCs were obtained from 10 healthy volunteers after oral informed consent. Twenty to 50 ml of peripheral blood was withdrawn from the vein of the forearm. PBMCs were isolated as described previously. Separation of monocytes from PBMC was conducted by counterflow centrifugal elutriation as described previously. Monocytes were suspended at a final con-centration of 1 × 106 cells/ml in RPMI 1640 medium (Nissui Co. Ltd., Tokyo, Japan) supplemented with 10% (v/v) heat-inactivated fetal calf serum, 20 Ag/ml of kanamycin, and 100 Ag/ml of streptomycin and penicillin (Sigma). Endotoxin concentrations of the medium described above were measured by the Endospecy (Seikagaku Kogyo, Tokyo, Japan) kit and found to be lower than the limit of detection of 0.06 EU/ml.
Cytokine assays Monocytes (1 × 106 cells/ml) were cultured with LPS, histamine, its agonists and antagonists, and dbcAMP at 37jC in a 5% CO2– air mixture. After 24 h of culture, the cell suspensions were transferred into eppendorf tubes and centrifuged. The cell-free supernatant fractions were assayed for IL-18 protein. IL-18 was measured using ELISA employing the multiple Abs sandwich principle (MBL, Nagoya, Japan). The detection limit of the ELISA for IL-18 was 10 pg/ml. The results were expressed as the mean F SEM of five donors.
Statistical analysis
The statistical significances were evaluated using ANOVA, followed by Dunnett’s test. A probability value less than 0.05 was considered to be significant.
Results
Time-course and dose-response relationship for the effects of LPS on IL-18 production in monocytes
LPS (1000 ng/ml) time-dependently induced IL-18 production. The production of IL-18 was significant at 18 h and reached maximum level at 24 h. Moreover, the effect of different concentrations (0 – 1000 ng/ml) of LPS on the changes in the production of IL-18 in the supernatant at 24 h was examined. LPS concentration-dependently induced IL-18 production. The level of IL-18 production reached to about 560 pg/ml with the stimulation of LPS (1000 ng/ml).
Effect of histamine, histamine receptor antagonists, agonists and dbcAMP on LPS-induced IL-18 production in monocytes .
The effect of exogenous histamine (0 – 10—4 M) on LPS (1000 ng/ml)-induced production of IL-18 was determined at 24 h after the start of culture. Histamine concentration-dependently inhibited IL-18 production. At 10—4 M, histamine completely abolished the production of IL-18. The IC50 value for the inhibitory effect of histamine on IL-18 production was estimated to be 500 nM. To determine the histamine receptor subtypes involved in histamine action, the effects of histamine receptor antagonists (0 – 10—4 M), including d-chlorpheniramine (H1), famotidine (H2), and thioperamide (H3 – H4), on the inhibitory effect of histamine (10—4 M) on IL-18 production were determined as the previous studies. Famotidine concentration-dependently antagonized the inhibitory effect of histamine on IL-18 production , whereas d-chlorpheniramine and thioperamide did not (data not shown). Another H2 receptor antagonist, ranitidine, also exerted a substantially similar effect to famotidine (data not shown). Selective H2 receptor agonist, dimaprit, mimicked the effects of histamine on IL-18 production. Moreover, another H2 receptor agonist, 4-methylhistamine, also mimicked that of histamine (data not shown). However, 2-pyridyl-ethylamine (H1-selective agonist) and R-(a)-methylhistamine (H3-agonist) had no effect. dbcAMP (cAMP-analog) concentration-dependently inhibited IL-18 production. At 10—4 M, the inhibition by dbcAMP was complete.
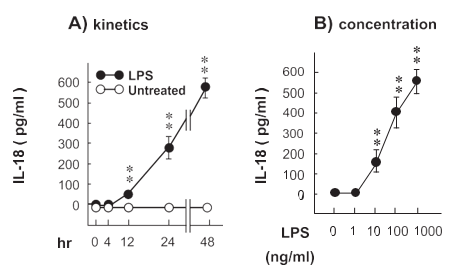
Fig. 1. The kinetics and dose-response relationship for the effect of LPS on IL-18 production in monocytes. (A) Monocytes (1 × 106 cells/ml) were incubated with LPS (1000 ng/ml) for 0 – 24 h. After the treatment, the level of IL-18 in the conditioned media was determined by ELISA. The results are the means F SEM of five donors. **P < 0.01 as compared with the corresponding value in 0 h. (B) Monocytes (1 × 106 cells/ml) were incubated with LPS for 24 h. **P < 0.01 as compared with the corresponding value in the absence of LPS. When an error bar was within a symbol, the bar was omitted.
Discussion
Histamine suppressed the expression of CD14 on monocytes via the H2 receptor, but had no effect on the expression of TLR-4. In the present study, we found that IL-18 could be detected in cultured medium of monocytes treated with more than 10 ng/ml of LPS. Histamine inhibited the production of IL-18 in LPS-treated monocytes . These results are consistent with the decrease in CD14 expression. The effect of histamine was blocked by H2-receptor antagonist, famotidine, but not by H1 and H3 – H4 receptor antagonists. In addition, H2 receptor agonists, 4-methylhistamine and dimaprit, mimicked the effects of histamine, but H1 and H3 receptor agonists did not. dbcAMP also inhibited IL-18 production. These results indicated that the effect of histamine on IL-18 production induced by LPS was mediated by the stimulation of H2 receptors and probably through the elevation of intercellular cAMP. LPS stimulates the production of histamine in various tissues through the induction of the histamine-forming enzyme, histidine decarboxylase, in mice. Therefore, we examined whether endogenous histamine might be involved in the regulation of basal production of IL-18. Any antagonists against H1, H2, and H3/H4 receptor had no effect on spontaneous IL-18 production (data not shown), suggesting the lack of involvement of endogenous histamine in the basal production of IL-18.
IL-18 is detected in mouse blood primed with Propionibacterium acnes and stimulated with LPS, as in vivo septic model. IL-18 observed in murine models of endotoxemia also induced lethal liver damage and splenocyte apoptosis. Administration of IL-18 in combination with IL-12 is capable of causing death in mice. Accordingly, therapeutic strategies aimed at decreasing IL-18 production may be beneficial in the treatment of severe trauma and sepsis. Some clinical trials have reported that administration of IFN-g to trauma and high-risk surgical patients will improve immune function, increase resistance to infection, and in some cases, enhance survival. However, IL-18 exerts its toxic activity through both IFN-gdependent and-independent mechanisms. Anti-IL-18 antibody was not able to protect mice against sepsis. In addition to the regulation of TNF-a production induced by LPS, the present study showed that histamine also inhibited LPS-induced IL-18 production through stimulation of H2 receptor and elevation of cAMP in monocytes.
It is well known that IL-18 is secreted from LPS activated monocytes or macrophages. In conclusion, we found that histamine inhibited LPS-induced IL-18 production in monocytes through stimulating of the H2 receptor. In the previous study, we found that the stimulation of H2 receptor suppressed IL-18-induced production of IL-12, TNF-a, and INF-g through inhibiting the cell-to-cell interaction between monocytes and T-cells. Thus, histamine moderates both LPSand IL-18-initiated cytokine production at different levels. In adult allergic asthmatic patients, the expression of CD14 on the surface of monocytes is upregulated by an allergen-dependent mechanism. However, the mechanism of histamine in regulating the expression of CD14 in asthma patients has not yet been established. Further study should be continued. All these findings as a whole indicated that pharmacological manipulation for stimulating H2 receptor might be a novel strategy for regulating the innate immune responses.