Keywords
Inflammation; Microglia; Ischemic stroke; Polarization; Lipoxin A4; Notch signaling pathway
Abstract
Microglia are rapidly activated after acute ischemic stroke, and the polarization of microglial is associated with the prognosis of acute ischemic stroke. Lipoxin A4 (LXA4), an anti-inflammatory agent, has a protective effect against ischemic stroke. However, the role of LXA4 on the polarization of microglial after acute ischemic stroke remains undetermined. We hypothesized that LXA4 may exert the neuroprotective effect though regulating the polarization of microglial. In this study, clinical features of acute ischemic stroke were simulated using a rat model of model of middle cerebral artery occlusion (MCAO) in vivo and the BV2 microglia oxygen-glucose deprivation/reoxygenation model (OGD/R) in vitro. The protective effects of LXA4 on cerebral ischemia-reperfusion injury were determined using TTC staining, HE staining, and TUNEL staining. The expression of targeted genes was assayed using quantitative real-time PCR (qRT-PCR), immunofluorescence, and western blot to investigated the regulation of LXA4 on microglia polarization after acute ischemic stroke. We found that LXA4 exerted protective effects on focal cerebral ischemia-reperfusion injury and reduced the expression of the proinflammatory cytokines IL-1β and TNF-α. Furthermore, LXA4 inhibited the expression of Notch-1, Hes1, iNOS and CD32 all of which are associated with the differentiation into M1 microglia. By contrast, LXA4 upregulated the expression of Hes5, Arg-1 and CD206 all of which are associated with M2 phenotype in microglia. In addition, blocking the Notch signaling pathway with the inhibitor DAPT significantly mitigated the effect of LXA4 on microglia differentiation. These data suggest that LXA4 may regulate the polarization of microglia after cerebral ischemia-reperfusion injury through the Notch signaling pathway.
Introduction
Ischemic stroke is a disease with high morbidity, disability rate, recurrence rate, and mortality. According to Latest research shows approximately 795,000 individuals experience a new or recurrent stroke each year, 610,000 of which are first attacks, and 185,000 are recurrent attacks, thus placing a heavy burden on families and society. To date, there are limited drugs with a proven therapeutic efficacy against acute ischemic stroke. Neuroprotection to prevent infarct progression as a potential treatment for acute ischemic stroke remains limited, and has been repurposed for stroke as an adjunct to mechanical thrombectomy. Therefore, identifying novel drugs that can effectively reduce infarct volume, delay stroke progression, and improve the prognosis in acute ischemic stroke remains warranted.
The causes of acute ischemic stroke are diverse, and its pathogenesis is complex. Various studies have shown that inflammatory responses play a pivotal role in the pathological mechanisms of acute ischemic stroke. Inflammatory reactions can, for instance, aggravate neuronal apoptosis. Microglia, the macrophage population of the central nervous system, is rapidly activated after acute ischemic stroke. Activated microglia have two polarization states, namely M1 and M2, which play a dual role in promoting and alleviating inflammation. The M1 phenotype secretes various inflammatory mediators and cytotoxic substances (e.g., tumor necrosis factor [TNF]-α, interleukin [IL]-1, and inducible nitric oxide synthase [iNOS]) aggravating the damage in the affected neural tissue, whereas the M2 phenotype can phagocytose cell fragments and secrete neurotrophic and anti-inflammatory factors (e.g., IL-10, arginase-1 [Arg-1], and brain-derived neurotrophic factor) to promote the repair of the damaged tissue. Since the two microglial activated states can shift between each other, drugs intervention specifically designed to enhance neuroprotective effects by converting microglia to M2 phenotype may have therapeutic benefits for acute ischemic stroke. The regulation of microglial polarization has been extensively investigated as potential therapeutic strategy against acute ischemic stroke with the aim to reduce the pro-inflammatory response and facilitate the repair of tissue injury.
In the central nervous system, the Notch signaling pathway is involved in dynamic control ranging from the reorganization of cell structures to functions of the nervous system. Once the Notch signaling pathway is activated, the Notch intracellular domain is released into the cytosol and translocated into the nucleus, thereby promoting the production of transcription activators and inducing downstream target genes of the Notch pathway including Hes1, Hes5, and NF-κB. Several studies have shown that the Notch signaling pathway is closely associated with microglia activation and polarization, as well as to the development and progression of central nervous system diseases. Thus, the Notch pathway may be an important target to regulate activation, polarization, and inflammatory responses of microglial cells.
Lipoxins (LXs) are arachidonic acid metabolites with strong pro- and anti-inflammatory effects. Lipoxin A4 (LXA4) is one of the important physiologically active forms of LXs, which binds to G protein-coupled receptor ALX with high affinity (also termed FPRL1/FPR2). The intraventricular injection of LXA4 after acute ischemic stroke can significantly reduce neurological impairment, infarct volume, permeability of the blood-brain barrier, and release of inflammatory factors. We have previously demonstrated that LXA4 can regulate LPS-induced BV2 microglial activation and differentiation via the Notch signaling pathway; however, ischemia-reperfusion injury is different from LPS induced inflammatory response, which better reflects the pathophysiological mechanism of ischemic stroke. Whether LXA4 can regulate microglial polarization after acute ischemic stroke to achieve anti-inflammatory effects and reduce inflammation-induced damage and whether the Notch signaling pathway is involved in this mechanism have yet to be determined.
In this study, we investigated the effects of LXA4 on microglial polarization after cerebral ischemia-reperfusion injury in an in vivo animal model of middle cerebral artery occlusion (MCAO), as well as in vitro in BV2 microglia using an oxygen-glucose deprivation/reoxygenation (OGD/R) model, to explore the underlying mechanisms of the LXA4 effects in ischemia and to provide a theoretical basis for novel clinical treatment options against ischemic stroke.
Materials and methods
Rat model of middle cerebral artery occlusion
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The in vivo experiments used 60 healthy male adult Sprague-Dawley rats (provided by the Henan Animal Experimental Animal Center) weighing 250–280 g. Animals dying before the chosen endpoint, no infarction except for sham were replaced to ensure at least n = 6 per group for quantification.
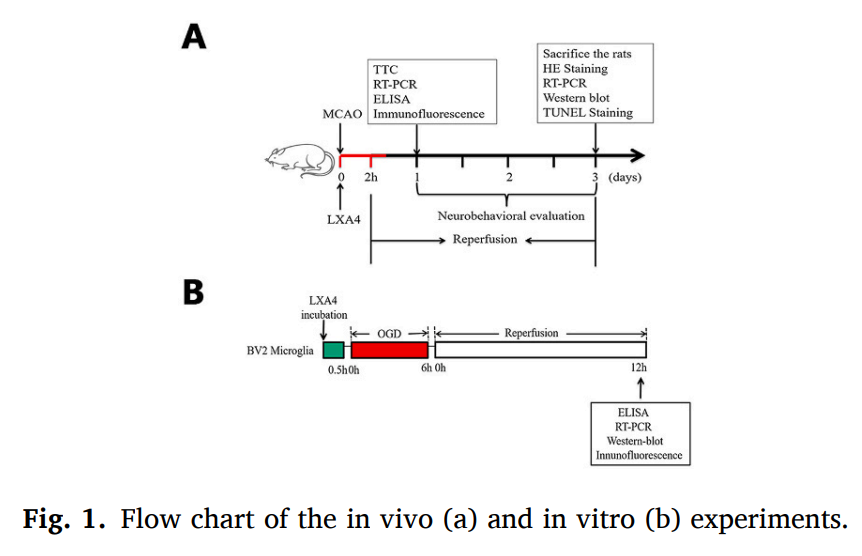
The MCAO model was slightly modified from that of a previously published method. In brief, the left common, external (ECA), and internal (ICA) carotid arteries were fully exposed and carefully dissected. After the ligature of the distal end of the ECA and its branches, a silicone-coated nylon monofilament (0.36 mm; #2636A4, Beijing Cinontech Co., Ltd., China) was inserted through the ECA stump into the ICA and gently advanced to block the middle cerebral artery. After 2 h of MCAO, the filament was removed to restore cerebral perfusion. The body temperature was maintained at 37 ℃ ± 0.5 ℃ with a heating lamp throughout the surgery and occlusion period. In the Sham group, all surgical procedures were the same as above except for the insertion of the nylon monofilament.
Drug treatment of the experimental animals
The experimental procedures of the in vivo experiments are shown in the figure. All the Sprague-Dawley rats were randomly assigned to four groups: (1) Sham, (2) MCAO, (3) MCAO +Vehicle, and (4) MCAO+LXA4.
An ethanol stock solution of LXA4 (5S,6R,15R-trihydroxy-7,9,13-trans-11-cis-eicosatetraenoic acid; 171030-11-8, Cayman) was stored at − 80 ℃ in the dark. In the present study, according to the previous experiment, a small amount of ethanol had no effect on cerebral infarction volume and nerve function and physiological saline was used as the control solvent, and the LXA4 stock solution was diluted in physiological saline to 0.2 mmol/L. Immediately after the establishment of the MCAO model, 1 nmol LXA4 (5 μL, 0.2 mmol/L) or 5 μL saline was injected slowly into the lateral ventricle using a 5-μL microinjector under the guidance of a stereotactic instrument (coordinates: anteroposterior, − 0.8 mm; lateral, 1.5 mm; dorsoventral, − 3.5 mm). Previous studies have shown that 100 nmol of LXA4 injected into the lateral ventricle of a rat exerts neuroprotective effects in ischemic stroke. Therefore, our study used this LXA4 concentration in the in vivo experiments. All surgical procedures were performed under aseptic conditions, and penicillin (200,000 U, intramuscular) was injected after the surgery to prevent infections.
BV2 microglial cell culture and LXA4 treatment
The BV2 murine microglial cell line was purchased from the Wuhan University China Culture Collection. In agreement with our previous experiments, BV2 microglia were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 supplemented with 10% fetal bovine serum and maintained in a humidified atmosphere of 5% CO2 at 37 ℃.
Our previous study comparing the effects of 1, 10, and 100 nmol/L LXA4 on BV2 cells in vitro suggests that the strongest anti-inflammatory effect was achieved at a concentration of 100 nmol/L. Therefore, we selected this concentration for the in vitro experiments of the present study. The BV2 microglial cells were divided into four groups: (1) Control, (2) LXA4, (3) OGD/R, and (4) OGD/R + LXA4. The basic procedure of these in vitro experiments is shown in the figure. And in the section of LXA4 regulates Notch signaling pathway, we pretreatment the DAPT (N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butylester) (Sigma-Aldrich, München, Germany) with a final concentration of 10 μM for 1 h to block the Notch signaling pathway in vitro experiment. The BV2 microglial cells were divided into five groups again: (1) Control, (2) OGD/R, (3) DAPT+OGD/R, (4) LXA4 + OGD/R, (5) DAPT+LXA4 + OGD/R.
Oxygen–glucose deprivation and reoxygenation
The OGD induction mimicking ischemic injury in BV2 cells was based on a previously reported method with minor modifications. For the OGD experiments, BV2 cells in the logarithmic growth phase were selected, the culture medium was changed to glucose-free DMEM/F12, and cells were transferred into a sealed chamber that was aerated with a 95% N2 / 5% CO2 gas mix (20 min, 1.5 L/min) to reduce the oxygen concentration. This chamber was then transferred into an incubator (37 ℃, 6 h, 2 L/min). For reoxygenation, the medium was replaced with glucose-containing DMEM/F12, and the cells were exposed to normal atmospheric conditions in an incubator. In our in vitro experiments, 6 h OGD and 12 h reoxygenation were selected as the optimal conditions for OGD/R in BV2 cells. BV2 microglia were incubated with LXA4 at 100 nmol/L for 30 min prior to OGD induction, and the supernatants and BV2 microglial cells of each group were collected for subsequent experiments.
Infarct and edema volume measurements
The 2,3,5-triphenyltetrazolium chloride (TTC) assay was performed 24 h after MCAO as described previously. Images were obtained using a digital camera by a researcher blinded to the group conditions used Adobe Photoshop CS6 software to measure infarct and edema volumes. The formulas were defined as follows: infarct volume ratio = [ipsilateral infarct volume – (ipsilateral brain volume – contralateral brain volume)] / contralateral brain volume × 100%, edema ratio = (ipsilateral brain volume – contralateral brain volume) / contralateral brain volume × 100%.
Evaluation of neurological deficits
Neurological scores were assessed by a blinded observer on days 1, 2, and 3 after MCAO. Scores from 0 to 5 were given based on a motor behavioral test as reported previously: 0, no neurological deficit; 1, failure to fully extend the contralateral forepaw; 2, decreased grip of the contralateral forelimb while the tail is pulled; 3, spontaneous circling or walking toward the contralateral side; 4, no spontaneous motor activity; 5, unresponsive to stimulation.
Quantitative real-time PCR (qRT-PCR)
qRT-PCRs were performed as described previously. In short, total RNA from the rat ischemic penumbra tissue or BV2 microglia was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and reverse-transcribed into cDNA using HiScript II Q RT SuperMix for qPCR (+ gDNA wiper; Vazyme Biotech Co., Ltd.). The qRT-PCR was performed using the AceQ® qPCRSYBR® GreenMaster Mix kit (Vazyme Biotech Co., Ltd.). GAPDH was used as an internal control, CT values represent real-time PCR values, and the 2-ΔΔCT method was used to calculate relative changes in gene expression.
Enzyme-linked immunosorbent assays (ELISAs)
The concentrations of IL-1β, TNF-α, IL-6 and IL-10 in brain ischemic penumbra or serum samples from rats and cell supernatants were determined using ELISA kits, according to the manufacturer’s instructions (Shanghai ExCell Biotechnology). The optical density at 450 nm was calculated by subtracting the background, and standard curves were plotted. The rats in each group were subjected to blood collection in the posterior orbital venous plexus at 24 h after MCAO. The culture supernatants of BV2 microglia were collected from each group at 12 h after OGD/R.
Immunofluorescence
Fixed brain tissue slices or cells were incubated overnight at 4 ℃ with antibodies directed against Iba1 (1:100; Abcam), iNOS (1:200; Proteintech Group, Inc.), CD32 (1:200; Thermo Fisher Scientific), Arg-1 (1:100; Santa Cruz Biotechnology), or CD206 (1:100; Abcam). The sections were then treated with fluorescent secondary antibodies (1:500; Abcam) for 1 h at room temperature. Sections were washed with phosphate-buffered saline and covers lipped with Prolong Antifade medium containing 4′,6-diamidino-2-phenylindole (DAPI; Sigma). Images were captured using a fluorescence microscope.
TUNEL staining
To analyze neuronal apoptosis, immunohistochemistry using mouse antibodies against NeuN (1:200; Abcam) was combined with terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL). Staining was performed using the In Situ Cell Death Detection Kit (Roche) according to the manufacturer’s instructions at 72 h post MCAO. Cells double-positive for TUNEL and NeuN were considered apoptotic neurons.
Hematoxylin-eosin (HE) staining
At 72 h after MCAO, samples from each experimental group were stained with HE. After euthanizing the rats with inhalation anesthetics, the whole brain was rapidly removed and fixed overnight in 4% paraformaldehyde. The brain tissue was then equilibrated in phosphatebuffered 30% sucrose solution, embedded in paraffin, and cut into coronal sections for HE staining. The morphological changes of the rat brain tissue were observed under a light microscope.
Western blotting
Protein expression levels of iNOS, Arg-1, Notch-1, Hes1, and Hes5 were measured using western blots. Total protein was extracted from the rat cerebral ischemic penumbra tissue or BV2 microglial cells. Western blotting analysis was conducted in accordance with our previous methods. The following primary antibodies were used for the western blots: Rabbit anti-iNOS (1:200; Proteintech Group, Inc.), mouse anti-Arg-1 (1:300; Santa Cruz Biotechnology), rabbit anti-Notch-1 (1:300; Proteintech Group, Inc.), mouse anti-Hes1 (1:500; Santa Cruz Biotechnology), rabbit anti-Hes5 (1:300; Zen BioScience Co), and mouse anti-tubulin (1:2000; Tianjin Sungene Biotechnology Co.), and horseradish peroxidase (HRP) -labeled goat anti-rabbit or goat anti-mouse secondary antibody (1:8000, Tianjin Sungene Biotechnology Co.). The reaction was observed using the chemiluminescence reagents provided by an ECL kit (Beyotime Biotechnology). Protein bands were quantified using the ImageJ software, and the band intensity was normalized to the band intensity of tubulin.
Flow Cytometry
BV2 microglia cells were seeded at 5 × 10/well and accordingly treated. Following treatments, cells were collected and washed three times with PBS, blocked with 0.1% Triton X-100 and 3% BSA in PBS, and stained with CD32-FITC and CD206-APC antibodies (Biolegend, San Diego, CA, USA) on ice in the dark for 30 min. After washing twice with PBS, cells were resuspended with 500 μL PBS and immediately observed using a FACS Calibur flow cytometer (BD Biosciences, USA); approximately 10,000 cells of each sample were collected and analyzed using FlowJo V10 software.
Statistical analysis
Data were expressed as the mean ± standard deviation (SD). Differences between two groups were analyzed using Student’s two-tailed t-test, while comparisons across multiple groups were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. Differences in neurological scores over time among the four groups were analyzed using two-way repeated measures ANOVA with Dunnett multiple comparison post hoc test. All statistical analyses were conducted using SPSS 22.0 software (SPSS Inc., USA), while figures were generated using GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, USA). Differences were considered statistically significant at P < 0.05.
Results
LXA4 protects against ischemic brain injury in MCAO rats
At 24 h after MCAO in rats, the brain tissue samples of the four experimental groups were stained with TTC. The TTC staining of normal brain tissue was demarcated by red, whereas the infarct area was pale. The Sham group did not show any obvious signs of infarction in the brain tissue. Compared with the Sham group, the volume of cerebral infarction was significantly increased in the MCAO group (infarct volume ratio: 49.00 ± 1.58%, P < 0.001). LXA4 administration influenced the infarct volume; in comparison to the MCAO+Vehicle group, the cerebral infarct volume was reduced in the MCAO+LXA4 group (infarct volume ratio: 26.67 ± 4.67%, P < 0.05). The edema quantification revealed analogous observations. Compared with the Sham group, the edema volume in the MCAO group was significantly increased (edema volume ratio: 16.17% ± 1.92%, P < 0.001). Similar to the LXA4 effect on the infarct volume, the edema volume in the MCAO+LXA4 group was significantly reduced compared to the MCAO+Vehicle group (edema volume ratio: 8.67 ± 1.63%, P < 0.05).
At 72 h after MCAO, tissue samples from each experimental group were also stained with HE. HE staining can show the infarct volume and the degree of injury in the brain tissue. The nerve fibers in the whole-brain coronal sections of the Sham group were evenly arranged, the neurons were abundant, the morphology was normal, the staining was uniform, and no obvious abnormality was observed. Infarcts were found in the cerebral cortex and the striatum of the MCAO+Vehicle and MCAO+LXA4 groups, and the HE staining was faint in the infarct regions. In HE-stained slices, the infarct area was reduced in the group exposed to LXA4 compared to the MCAO group without drug treatment. At 20× magnification, the infarct area appeared softened in the cortex and hippocampus, and the structures were loosely arranged. Several neuronal cells were necrotic forming cavities, and their nuclei were deflated, intensely stained and shrinkage. Moreover, cellular infiltrations and occasional local oozing from blood vessels were observed. In the group with LXA4 administration, the infarct volume, neuronal necrosis, and inflammatory cell infiltration were significantly decreased compared with the MCAO group without drug treatment.
We performed neurological function scores on the 1st, 2nd, and 3rd day after focal cerebral ischemia-reperfusion injury in rats. Compared with the Sham group, the MCAO group and the MCAO+Vehicle group had higher scores and showed significant neuromotor dysfunction. Most of the right forelimbs were abducted when the tail was raised. When walking, the tail was centered on the right side, and even a part of it showed loss of consciousness and no spontaneous activity. After LXA4 intervention, although there was no significant difference between the MCAO+LXA4 group and MCAO+Vehicle group of the behavioral scores on day 1 and day 2 (P > 0.05), the neurological function significantly improved and the behavioral score decreased on the day 3 (P < 0.05).
The LXA4 effects on neuronal apoptosis were also observed using fluorescent double-staining for NeuN and TUNEL in brain slices 72 h after MCAO. NeuN and TUNEL double-positive cells were counted as apoptotic neurons and expressed as TUNEL++NeuN+. The statistical analysis of the TUNEL-positive neurons showed that the percentage (TUNEL++NeuN+) / NeuN+ was significantly higher in the MCAO group than in the Sham group (P < 0.001). After LXA4 administration, this percentage was significantly lower in the MCAO+LXA4 group than in the vehicle-treated MCAO group (P < 0.05). The results from the TUNEL staining further confirmed that LXA4 treatment prevented ischemia-induced neuronal apoptosis.
Effects of LXA4 on microglia activation after cerebral ischemia-reperfusion injury
We used immunofluorescence to observe the effect of LXA4 on microglia activation after cerebral ischemia-reperfusion injury. Iba1 was used as a marker for microglia. At 24 h after MCAO, it was apparent that almost no expression of microglia was observed in the sham group. Interestingly, microglia were significantly activated after MCAO and were heavily labeled by Iba1, whereas LXA4 significantly inhibited microglia expression after intervention.
At 12 h following BV2 microglia exposure to OGD/R, microglia cells were highly branched. In activated microglia, part of the cell bodies became larger and rounder, while protrusions decreased in number and became thicker.
LXA4 inhibits inflammatory responses after MCAO and OGD/R
The secretion of pro-inflammatory factors promotes brain tissue damage after an ischemic stroke. Previous studies have shown that LXA4 has protective effects on cerebral ischemia-reperfusion injury and regulates changes in inflammatory factors after ischemia. To observe the effects of LXA4 on inflammatory cytokines after microglial activation, qRT-PCRs and ELISAs were used to detect inflammatory factors at the levels of genes and proteins, respectively.
At 24 h after MCAO, total RNA was extracted from the ischemic penumbra for qRT-PCRs. The mRNA expression levels of TNF-α and IL-1β were increased 24 h after MCAO in comparison to the Sham group (both P < 0.001), whereas they significantly decreased in the group exposed to LXA4 treatment. After LXA4 administration, the mRNA expression levels of IL-1β and TNF-α in the MCAO+LXA4 group were significantly lower than those in the MCAO+ Vehicle group.
The mRNA expression of IL-1β and TNF-α was also assessed by qRT-PCR in BV2 microglia 12 h after OGD/R. OGD/R induced a significant increase in IL-1β and TNF-α mRNA compared to the Control group (P < 0.01). After administration of LXA4, the mRNA expression levels of IL-1β and TNF-α were decreased in the OGD/R + LXA4 group compared with those in the OGD/R+ Vehicle group (P < 0.05).
Additionally, ELISAs were used to detect the protein levels of IL-1β and TNF-α in the serum of rats 24 h after MCAO. Compared to the Sham group, the expression levels of IL-1β and TNF-α proteins were elevated in the MCAO group (P < 0.05 and P < 0.01, respectively). After LXA4 administration, the protein expression levels of IL-1β and TNF-α in the MCAO+LXA4 group were significantly lower than those in the MCAO+ Vehicle group (P < 0.05).
The ELISA method was also used with supernatants of BV2 microglia to detect changes in protein expression of IL-1β and TNF-α 12 h after OGD/R. Compared to the Control group, the expression of IL-1β and TNF-α protein in the OGD/R+ Vehicle group was increased (P < 0.05 and P < 0.01, respectively). After administration of LXA4, the expression levels of IL-1β and TNF-α in the OGD/R + LXA4 group were significantly lower than those in the OGD/R+ Vehicle group (both P < 0.05).
In summary, LXA4 inhibited after cerebral ischemia-reperfusion injury the expression of genes and proteins of the inflammatory factors IL-1β and TNF-α that are associated with M1-type microglia. However, no significant differences were observed in IL-10 expression levels among all groups (data have not shown).
LXA4 shifts after OGD/R and MCAO the microglial polarization from M1 to M2
Microglia can be rapidly activated after cerebral ischemia-reperfusion injury, and the phenotype of the activated microglia can be distinguished by its specific surface markers. To determine whether LXA4 influences the polarization phenotype in microglia, qRT-PCR, western blot, and immunofluorescence were employed in in vivo and in vitro experiments.
At 72 h after MCAO, qRT-PCRs demonstrated that the expression levels of M1 (iNOS, CD32) and M2 (Arg-1, CD206) phenotype markers were significantly increased in the MCAO group (P < 0.001, P < 0.001, P < 0.001, and P < 0.001, respectively). After administration of LXA4, the expression of the M1-type marker CD32 was decreased (P < 0.001), whereas the expression of iNOS was not significantly changed (P > 0.05). However, the expression levels of the M2-type markers Arg-1 and CD206 were significantly increased (P < 0.01 and P < 0.001, respectively).
In BV2 microglia, qRT-PCRs of samples 12 h after OGD/R showed that the expression levels of markers for the M1 (iNOS, CD32) and M2 (Arg-1, CD206) phenotypes were significantly increased in the OGD/R group (P < 0.01, P < 0.01, P < 0.01, and P < 0.01, respectively). In the group exposed to LXA4, the expression levels of the M1-type markers iNOS and CD32 were decreased (P < 0.05 and P < 0.01, respectively), whereas the levels of the M2-type markers Arg-1 and CD206 were significantly increased (P < 0.01 and P < 0.01, respectively).
Using western blots, changes in the levels of these proteins were also assessed. At 72 h after MCAO, the protein levels of the M1 marker iNOS and the M2 marker Arg-1 were significantly increased in the MCAO group compared with the Sham group, (P < 0.001 and P < 0.001, respectively). In the presence of LXA4, the expression of the M1 marker iNOS was inhibited at the protein level (P < 0.01), whereas the M2-type marker Arg-1 was increased compared with the MCAO+Vehicle group (P < 0.001).
At 12 h after OGD/R, the western blots of BV2 microglia showed that the protein levels of the M1-type marker iNOS and the M2-type marker Arg-1 were significantly increased in the OGD/R + Vehicle group compared with those in the Control group (P < 0.001 and P < 0.01, respectively). Administration of LXA4 decreased the protein expression of iNOS (P < 0.001), whereas the expression level of Arg-1 was increased (P < 0.05) in the OGD/R + LXA4 group compared with those in the OGD/R + Vehicle group.
Immunofluorescence staining in BV2 microglia further confirmed these qRT-PCR and western blot results regarding the examined markers. The expression of M1 (iNOS, CD32) and M2 (Arg-1, CD206) phenotype markers were significantly increased in the OGD/R group. After administration of LXA4, the expression levels of the M1 phenotype markers iNOS and CD32 were decreased, whereas those of the M2-type markers Arg-1 and CD206 were significantly increased. In addition, in vitro, we detected the expressions of CD32 and CD206 using flow cytometry. As shown in the figure the OGD/R injury caused a significant increase in the number of CD32+ cells in BV2 microglia, while LXA4 treatment increased the number of CD206+ cells and decreased that of CD32+ cells in OGD/R treated microglia.
The findings from both gene and protein levels strongly suggest that LXA4 can promote the M2 polarization of microglia after cerebral ischemia-reperfusion injury.
LXA4 may regulate microglial polarization after cerebral ischemia-reperfusion injury through the Notch signaling pathway
To investigate whether LXA4 influences microglial activation and differentiation via the Notch signaling pathway, Notch-1 (Notch receptor), Hes1, and Hes5 (downstream genes of the Notch pathway) were selected as targets for further experiments.
At 72 h after MCAO, qRT-PCRs revealed that the mRNA levels of Notch-1, Hes1, and Hes5 were significantly increased in the MCAO group (P < 0.001, P < 0.001, and P < 0.01, respectively). In the MCAO group exposed to LXA4, the expression levels of Notch-1 and Hes1 were decreased (P < 0.01 and P < 0.05, respectively), whereas Hes5 expression was significantly increased (P < 0.05).
In BV2 microglia, the mRNA levels of Notch-1, Hes1, and Hes5 were significantly increased in the OGD/R + Vehicle group (P < 0.01, P < 0.001, and P < 0.001, respectively) at 12 h after OGD/R. After LXA4administration, the expression levels of Notch-1 and Hes1 were decreased (P < 0.01 and P < 0.01, respectively), whereas that of Hes5 was significantly increased (P < 0.01).
Additionally, the protein levels were evaluated using western blots. Compared with the Sham group, the protein levels of Notch-1, Hes1, and Hes5 were significantly increased in the MCAO group 72 h following hypoxia (P < 0.001, P < 0.05, and P < 0.05, respectively). After LXA4 administration, the expression levels of Notch-1 and Hes1 were decreased (P < 0.001 and P < 0.05, respectively), whereas that of Hes5 was significantly increased (P < 0.001).
At 12 h after OGD/R, western blots of samples from BV2 microglia showed that the expression levels of Notch-1, Hes1, and Hes5 were significantly increased in the OGD/R + Vehicle group (P < 0.001, P < 0.001, and P < 0.001, respectively). In the OGD/R group treated with LXA4, the protein levels of Notch-1 and Hes1 were decreased (P < 0.05 and P < 0.05, respectively), whereas that of Hes5 was significantly increased (P < 0.01).
These results suggest that the Notch signaling pathway is activated after cerebral ischemia-reperfusion injury. Moreover, LXA4 may regulate the polarization of microglia through the Notch signaling pathway.
DAPT, a Notch signaling pathway inhibitor can significantly counteract the effect of LXA4 on microglia polarization
We investigated the mechanism underlaying LXA4 effects on microglia polarization and its anti-inflammatory effect by blocking Notch signaling pathway with the γ-secretase inhibitor, DAPT. Firstly, we evaluated the expression levels of Notch signaling pathway related proteins, including Notch1, Hes1, and Hes5, and the expression levels of the microglia M1 marker, iNOS, and the M2 marker, Arg-1, using western blot. We also evaluated the expression levels of the inflammatory cytokines, IL-1β, TNF-α, IL-6 and IL-10, after blocking the Notch signaling pathway. Our treatment groups were as follows: Control, OGD/R, DAPT+OGD/R, LXA4 + OGD/R, DAPT+LXA4 + OGD/R.
At 12 h after OGD/R treatment, the expression levels of Notch-1 and Hes1 in the OGD/R group were significantly higher than those of the control group, while that of Hes5 was not. In the OGD/R + DAPT treatment group, the expression level of Hes1 was significantly decreased (P<0.05), while no significant differences in the expression levels of Notch-1 or Hes5 were observed (P > 0.05). In the LXA4 pretreatment group, the expression levels of Notch-1 and Hes1 were significantly decreased (P < 0.05) while that of Hes5 was increased (P < 0.05). Furthermore, we observed that co-treatment of LXA4 and DAPT in the DAPT+LXA4 + OGD/R group significantly inhibited the expression of Notch-1, Hes1, and Hes5 compared to that observed in the LXA4 + OGD/R treatment group (P<0.05).
We also observed that pretreatment with LXA4 significantly suppressed the expression of iNOS but increase that of Arg-1 following OGD/R-induced activation and damage of BV2 microglia cells. However, co-treatment of LXA4 and DAPT counteracted the polarization effect of LXA4 on BV2 microglia cells.
The secretion of IL-1β, TNF-α, and IL-6 was increased, in comparison with the control group, in various degrees in response to OGD/R-induced damage of BV2 microglia cells, however, no significant differences in the levels of IL-10 were observed in all groups. LXA4 significantly reduced the secretion of pro-inflammatory cytokines, however, this effect was abolished by DAPT, which further suggests that LXA4 exerts its anti-inflammatory effects through the Notch signaling pathway.
Discussion
Ischemic stroke is a serious vascular disease that significantly threats public health. Due to progressive improvements in recent years regarding our understanding of its pathogenesis, it is now recognized that cerebral ischemia-reperfusion injury is a complex pathophysiological process involving inflammation, cellular damage by oxygen-derived free radicals, energy depletion in the brain, intracellular calcium ion overload, acidosis, toxicity of excitatory amino acids, nerve cell apoptosis, and breakdown of the blood-brain barrier.
Several studies have shown that inflammation plays a pivotal role in the development of ischemic stroke. Microglia play an important role in the inflammatory response, and the tissue surrounding the infarct often shows signs of microglial activation and increased levels of inflammatory factors. Microglia are equivalent to macrophages of the central nervous system, and have diverse functions such as support, nutrition, and immune surveillance. After activation, microglia mainly differentiate into M1 or M2 phenotypes. M1-type microglial cells are strongly associated with phagocytosis and cytotoxicity, and their corresponding molecular markers are iNOS and CD32, among others. Various inflammatory factors released by M1-type microglia mediate the inflammatory damage to nerve cells and neural tissue. M2-type cells have anti-inflammatory and nerve repair functions and are mainly involved in inhibition of inflammation, immune regulation, and tissue repair. Their molecular markers are Arg-1 and CD206, among others. M2 microglia can release chemokines, induce migration of microglial cells toward the lesion, and phagocytose harmful molecules and cell debris. M2-type microglia can also release brain-derived neurotrophic factor, vascular endothelial growth factor, and anti-inflammatory mediators to alleviate the inflammatory injury and promote resolution of the inflammation, tissue repair, and nerve regeneration. In addition, Arg-1 is upregulated in microglia of the M2 phenotype and can compete with iNOS for its substrate arginine, decrease nitric oxide levels, reduce tissue damage, promote repair of damaged tissue, and lay the foundation for tissue remodeling. Studies has indicated that after ischemic stroke, a large number of M2 microglia aggregates are found in the subventricular layer of the striatum. Moreover, the insulin-like growth factor-1 produced by these M2-type microglia is effective in promoting nerve cell regeneration. Another study injected IL-4 into the lateral ventricle of a mouse model of cerebral ischemia, which induced M2 microglia proliferation and significantly promoted long-term neurological recovery after cerebral ischemia. Other studies found that M2 microglia also play an important role in neovascularization and tissue remodeling after ischemic stroke. These studies demonstrate that regulation of microglial polarization can be beneficial, prevent harm, and protect the central nervous system from detrimental effects, thereby improving the prognosis in ischemic stroke.
The molecular signal transduction mechanisms of microglial polarization have emerged as a novel topic in recent years. The Notch signaling pathway, which may be important for the regulation of microglial polarization, is composed of the Notch receptor, Notch ligand, effector molecules, and regulatory factors. Previous studies have shown that elevated levels of the Notch-1 receptor and the downstream molecule Hes1 suggest the M1-type polarization in microglia, whereas upregulation of Hes5 levels is related to M2-type polarization. The Notch pathway may be important for regulating the activation and differentiation of microglia and the inflammatory response. Therefore, regulation of the Notch signaling pathway may affect the polarization state of microglial cells after ischemic stroke, which is expected to influence the disease course and improve the prognosis in ischemic stroke.
The LX family of mediators is the first endogenous “brake signal” found in the body with a strong anti-inflammatory effect and promotes the regression of inflammation. LXA4 is an important member of this family and binds to its specific G protein-coupled receptor to exert its anti-inflammatory effects. Previous studies have shown that picomolar doses of LXA4 can induce a substantial anti-inflammatory effect in vivo. Several studies have confirmed that LXA4 can protect against cerebral ischemia-reperfusion injury by reducing the production and expression of pro-inflammatory factors. However, whether LXA4 can regulate the polarization of microglia to achieve anti-inflammatory effects and reduce the inflammatory damage after acute ischemic stroke has yet to be determined. Moreover, it remains known whether these LXA4 effects involve Notch signaling.
In this study, a rat model of middle cerebral artery occlusion was established, and blood flow was restored 2 h after ischemia to simulate ischemia-reperfusion in stroke patients. Additionally, the immortalized murine microglial cell line BV2 was used as a model of inflammation. BV2 microglia retains many morphological, phenotypical, and functional characteristics of microglia in situ and is, thus, an ideal model to study microglia.
In the in vivo experiments, we first investigated the protective effects of LXA4 on cerebral ischemia-reperfusion injury in rats. LXA4 was injected into the lateral ventricle of the rats immediately after MCAO. After 24 h of reperfusion, there was no significant difference in the neurological deficit score between the MCAO+LXA4 and MCAO+Vehicle groups, but the TTC staining revealed group differences, in which LXA4 administration significantly reduced the infarct and edema volumes in rats. This was confirmed in HE staining with the reduction in infarct volume and inflammatory infiltration. This study found no significant differences in neurological impairment between the rats on the 1st and 2nd day. However, 72 h post reperfusion, the MCAO+LXA4 group significantly improved compared with the untreated group, while the percentage of TUNEL-positive neurons was significantly decreased after LXA4 treatment compared to the MCAO and MCAO+Vehicle groups. Thus, the LXA4 intervention significantly reduced the infarct size, and the TUNEL staining suggests that LXA4 has a protective effect in focal cerebral ischemia-reperfusion injury.
Previous studies have also shown that LXA4 has anti-inflammatory effect; hence we examined the expression of IL-1β and TNF-α using both qRT-PCR and ELISA in vivo and in vitro. The results of these experiments showed that activation of microglia after MCAO and OGD/R induced the expression of the pro-inflammatory factors IL-1β and TNF-α and that LXA4 administration significantly reduced the expression of these pro-inflammatory factors, indicating that LXA4 can inhibit their secretion by microglia, which may contribute to the observed anti-inflammatory effects. LXA4 exerted its effects by inhibiting the expression of pro-inflammatory factors rather than promoting the expression of anti-inflammatory factor IL-10 (data not shown).
At 72 h after MCAO and at 12 h after OGD/R in BV2 microglia, qRT-PCRs, western blots, and immunofluorescence assays showed that compared with the resting state, the expression levels of the M1-type markers iNOS and CD32 and M2-type markers Arg-1 and CD206 were increased. After LXA4 administration, the expression of the M1 markers iNOS and CD32 was downregulated, whereas the levels of the M2 markers Arg-1 and CD206 were increased compared to the untreated groups. The above findings confirmed that LXA4 can regulate the polarization of microglia at the gene and protein levels and that LXA4 inhibits the expression of microglial M1 phenotype markers while simultaneously increasing the expression of M2 markers.
The present study also found that LXA4 can affect downstream effector molecules of the Notch signaling pathway at the gene and protein levels. The reductions in Notch-1 and Hes1 expression levels are associated with M1-type polarization of microglial cells, whereas upregulation of Hes5 levels is related to M2-type polarization, suggesting that LXA4 promotes the transformation of microglia from the M1 to the M2 phenotype through the Notch signaling pathway. γ-secretase, an essential enzyme in the activation of Notch signaling pathway, rapidly cleaves Notch following ligand mediated removal of Notch extracellular domain. Therefore, γ-secretase inhibitors, such as DAPT, can block the Notch signaling pathway, thereby ameliorating the effects of LXA4 on microglia and neuroprotective effect, which further demonstrates that LXA4 regulates the polarization of BV2 microglia through the Notch signaling pathway.
In summary, the results of this study demonstrate that LXA4 can inhibit the pro-inflammatory response after ischemic stroke and exert a substantial neuroprotective effect. More importantly, this study illustrates, to the best of our knowledge, for the first time that LXA4 can regulate the polarization of microglia after ischemic stroke by shifting them from the M1 to M2 phenotype through the Notch signaling pathway, which provides insights into the potential use of LXA4 as a novel treatment strategy of ischemic strokes. Although LXA4 has a strong anti-inflammatory effect as an endogenous anti-inflammatory lipid mediator, in-depth research regarding its mechanisms is remains warranted prior to any clinical application.